-
- Art. 3 FC
- Art. 5a FC
- Art. 6 FC
- Art. 10 FC
- Art. 13 FC
- Art. 16 FC
- Art. 17 FC
- Art. 20 FC
- Art. 22 FC
- Art. 26 FC
- Art. 29a FC
- Art. 30 FC
- Art. 31 FC
- Art. 32 FC
- Art. 42 FC
- Art. 43 FC
- Art. 43a FC
- Art. 45 FC
- Art. 55 FC
- Art. 56 FC
- Art. 60 FC
- Art. 68 FC
- Art. 74 FC
- Art. 75b FC
- Art. 77 FC
- Art. 81 FC
- Art. 96 para. 1 FC
- Art. 96 para. 2 lit. a FC
- Art. 110 FC
- Art. 117a FC
- Art. 118 FC
- Art. 123a FC
- Art. 123b FC
- Art. 130 FC
- Art. 136 FC
- Art. 164 FC
- Art. 166 FC
- Art. 170 FC
- Art. 178 FC
- Art. 189 FC
- Art. 191 FC
-
- Art. 11 CO
- Art. 12 CO
- Art. 50 CO
- Art. 51 CO
- Art. 84 CO
- Art. 97 CO
- Art. 98 CO
- Art. 99 CO
- Art. 100 CO
- Art. 143 CO
- Art. 144 CO
- Art. 145 CO
- Art. 146 CO
- Art. 147 CO
- Art. 148 CO
- Art. 149 CO
- Art. 150 CO
- Art. 633 CO
- Art. 701 CO
- Art. 715 CO
- Art. 715a CO
- Art. 734f CO
- Art. 785 CO
- Art. 786 CO
- Art. 787 CO
- Art. 788 CO
- Art. 808c CO
- Transitional provisions to the revision of the Stock Corporation Act of June 19, 2020
-
- Art. 2 PRA
- Art. 3 PRA
- Art. 4 PRA
- Art. 6 PRA
- Art. 10 PRA
- Art. 10a PRA
- Art. 11 PRA
- Art. 12 PRA
- Art. 13 PRA
- Art. 14 PRA
- Art. 15 PRA
- Art. 16 PRA
- Art. 17 PRA
- Art. 19 PRA
- Art. 20 PRA
- Art. 21 PRA
- Art. 22 PRA
- Art. 23 PRA
- Art. 24 PRA
- Art. 25 PRA
- Art. 26 PRA
- Art. 27 PRA
- Art. 29 PRA
- Art. 30 PRA
- Art. 31 PRA
- Art. 32 PRA
- Art. 32a PRA
- Art. 33 PRA
- Art. 34 PRA
- Art. 35 PRA
- Art. 36 PRA
- Art. 37 PRA
- Art. 38 PRA
- Art. 39 PRA
- Art. 40 PRA
- Art. 41 PRA
- Art. 42 PRA
- Art. 43 PRA
- Art. 44 PRA
- Art. 45 PRA
- Art. 46 PRA
- Art. 47 PRA
- Art. 48 PRA
- Art. 49 PRA
- Art. 50 PRA
- Art. 51 PRA
- Art. 52 PRA
- Art. 53 PRA
- Art. 54 PRA
- Art. 55 PRA
- Art. 56 PRA
- Art. 57 PRA
- Art. 58 PRA
- Art. 59a PRA
- Art. 59b PRA
- Art. 59c PRA
- Art. 60 PRA
- Art. 60a PRA
- Art. 62 PRA
- Art. 63 PRA
- Art. 64 PRA
- Art. 67 PRA
- Art. 67a PRA
- Art. 67b PRA
- Art. 73 PRA
- Art. 73a PRA
- Art. 75 PRA
- Art. 75a PRA
- Art. 76 PRA
- Art. 76a PRA
- Art. 90 PRA
-
- Art. 1 IMAC
- Art. 1a IMAC
- Art. 3 para. 1 and 2 IMAC
- Art. 8 IMAC
- Art. 8a IMAC
- Art. 11b IMAC
- Art. 16 IMAC
- Art. 17 IMAC
- Art. 17a IMAC
- Art. 32 IMAC
- Art. 35 IMAC
- Art. 47 IMAC
- Art. 63 IMAC
- Art. 67 IMAC
- Art. 67a IMAC
- Art. 74 IMAC
- Art. 74a IMAC
- Art. 80 IMAC
- Art. 80a IMAC
- Art. 80b IMAC
- Art. 80c IMAC
- Art. 80d IMAC
- Art. 80h IMAC
-
- Vorb. zu Art. 1 FADP
- Art. 1 FADP
- Art. 2 FADP
- Art. 3 FADP
- Art. 4 FADP
- Art. 5 lit. c FADP
- Art. 5 lit. d FADP
- Art. 5 lit. f und g FADP
- Art. 6 para. 3-5 FADP
- Art. 6 Abs. 6 and 7 FADP
- Art. 7 FADP
- Art. 10 FADP
- Art. 11 FADP
- Art. 12 FADP
- Art. 14 FADP
- Art. 15 FADP
- Art. 18 FADP
- Art. 19 FADP
- Art. 20 FADP
- Art. 22 FADP
- Art. 23 FADP
- Art. 25 FADP
- Art. 26 FADP
- Art. 27 FADP
- Art. 31 para. 2 lit. e FADP
- Art. 33 FADP
- Art. 34 FADP
- Art. 35 FADP
- Art. 38 FADP
- Art. 39 FADP
- Art. 40 FADP
- Art. 41 FADP
- Art. 42 FADP
- Art. 43 FADP
- Art. 44 FADP
- Art. 44a FADP
- Art. 45 FADP
- Art. 46 FADP
- Art. 47 FADP
- Art. 47a FADP
- Art. 48 FADP
- Art. 49 FADP
- Art. 50 FADP
- Art. 51 FADP
- Art. 52 FADP
- Art. 54 FADP
- Art. 55 FADP
- Art. 57 FADP
- Art. 58 FADP
- Art. 60 FADP
- Art. 61 FADP
- Art. 62 FADP
- Art. 63 FADP
- Art. 64 FADP
- Art. 65 FADP
- Art. 66 FADP
- Art. 67 FADP
- Art. 69 FADP
- Art. 72 FADP
- Art. 72a FADP
-
- Art. 2 CCC (Convention on Cybercrime)
- Art. 3 CCC (Convention on Cybercrime)
- Art. 4 CCC (Convention on Cybercrime)
- Art. 5 CCC (Convention on Cybercrime)
- Art. 6 CCC (Convention on Cybercrime)
- Art. 7 CCC (Convention on Cybercrime)
- Art. 8 CCC (Convention on Cybercrime)
- Art. 9 CCC (Convention on Cybercrime)
- Art. 11 CCC (Convention on Cybercrime)
- Art. 12 CCC (Convention on Cybercrime)
- Art. 16 CCC (Convention on Cybercrime)
- Art. 18 CCC (Convention on Cybercrime)
- Art. 25 CCC (Convention on Cybercrime)
- Art. 27 CCC (Convention on Cybercrime)
- Art. 28 CCC (Convention on Cybercrime)
- Art. 29 CCC (Convention on Cybercrime)
- Art. 32 CCC (Convention on Cybercrime)
- Art. 33 CCC (Convention on Cybercrime)
- Art. 34 CCC (Convention on Cybercrime)
-
- Art. 2 para. 1 AMLA
- Art. 2a para. 1-2 and 4-5 AMLA
- Art. 2 para. 3 AMLA
- Art. 3 AMLA
- Art. 7 AMLA
- Art. 7a AMLA
- Art. 8 AMLA
- Art. 8a AMLA
- Art. 11 AMLA
- Art. 14 AMLA
- Art. 15 AMLA
- Art. 20 AMLA
- Art. 23 AMLA
- Art. 24 AMLA
- Art. 24a AMLA
- Art. 25 AMLA
- Art. 26 AMLA
- Art. 26a AMLA
- Art. 27 AMLA
- Art. 28 AMLA
- Art. 29 AMLA
- Art. 29a AMLA
- Art. 29b AMLA
- Art. 30 AMLA
- Art. 31 AMLA
- Art. 31a AMLA
- Art. 32 AMLA
- Art. 38 AMLA
FEDERAL CONSTITUTION
MEDICAL DEVICES ORDINANCE
CODE OF OBLIGATIONS
FEDERAL LAW ON PRIVATE INTERNATIONAL LAW
LUGANO CONVENTION
CODE OF CRIMINAL PROCEDURE
CIVIL PROCEDURE CODE
FEDERAL ACT ON POLITICAL RIGHTS
CIVIL CODE
FEDERAL ACT ON CARTELS AND OTHER RESTRAINTS OF COMPETITION
FEDERAL ACT ON INTERNATIONAL MUTUAL ASSISTANCE IN CRIMINAL MATTERS
DEBT ENFORCEMENT AND BANKRUPTCY ACT
FEDERAL ACT ON DATA PROTECTION
CRIMINAL CODE
CYBERCRIME CONVENTION
COMMERCIAL REGISTER ORDINANCE
FEDERAL ACT ON COMBATING MONEY LAUNDERING AND TERRORIST FINANCING
FREEDOM OF INFORMATION ACT
FEDERAL ACT ON THE INTERNATIONAL TRANSFER OF CULTURAL PROPERTY
I. General
1 Art. 45 para. 7 TPA authorizes the Federal Council to regulate the reprocessing and reuse of single-use devices and to explicitly declare these activities to be permissible. Even if the TPA itself does not contain an explicit prohibition on the reprocessing of used single-use devices, this prohibition arises implicitly from Art. 45 para. 7 TPA. Whether this implicit prohibition of the reprocessing of used single-use devices and the Art. 73 para. 1 MedDO issued on this basis are sufficient from a constitutional point of view – in particular since violation of Art. 73 para. 1 MedDO is punishable by law – may at least be questioned.
2 If the Federal Council declares the reprocessing and reuse of single-use devices permissible on the basis of Art. 45 para. 7 TPA, it must also define the corresponding requirements. Currently, the Federal Council has not made use of its authority under Art. 45 para. 7 TPA, because Art. 73 para. 1 MedDO explicitly stipulates that the reprocessing and reuse of used single-use devices is prohibited.
3 Before the revised Medical Devices Ordinance came into force, the reprocessing of single-use devices was not explicitly regulated by law. In practice, single-use devices were often reprocessed and reused, especially by hospitals, not least for cost reasons. The law at that time basically allowed the reprocessing of single-use devices, provided that the health of patients was not endangered and that the requirements for the initial placing on the market were also met for the reprocessed single-use device. In addition, the placing on the market as single-use devices by the manufacturers was often seen as economically motivated (and not as a result of increased patient protection), which is why the manufacturers' instructions in this regard were often ignored. However, with the introduction of Art. 73 MedDO, the reprocessing and reuse of single-use devices was prohibited by law and made punishable (Art. 86 para. 1 let. d TPA).
II. Para. 1
4 According to Art. 73 para. 1 MedDO, the reprocessing and reuse of single-use devices is prohibited. Any procedure to which a used medical device is subjected so that it can be reused safely is deemed to be reprocessing. These procedures include cleaning, disinfection, sterilization and similar procedures, in particular packaging, transport and storage, as well as testing and restoring the technical and functional safety of the used product (Art. 4 para. 1 let. e MedDO). However, the term “reprocessing” as defined in Art. 73 para. 1 MedDO does not apply to cases in which a new (single-use) device must be cleaned, disinfected, sterilized or subjected to similar procedures prior to its first use. In this case, it is referred to as preparation for first use, which is part of maintenance (see Art. 4 para. 1 let. d MedDO). Since preparation for first use is part of maintenance, the provisions of Art. 71 MedDO apply.
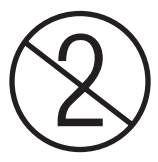
5 However, the MedDO does not contain a separate definition of the term “single-use device”, which is why the definition in Regulation (EU) 2017/745 of the European Parliament and of the Council of April 5, 2017 on medical devices (“EU MDR”) applies by virtue of the reference in Art. 4 para. 2 MedDO. A device that is intended for use on a single occasion is therefore designated as a single-use device (Art. 2 no. 8 EU MDR). Examples of single-use devices include disposable drill heads, electrophysiology catheters or ultrasonic scissors, but also simple products such as surgical gloves, cannulas, infusion tubes or plasters. Whether a medical device is a single-use device or can be used multiple times is defined by the intended use and the manufacturer. A single-use device is usually marked with the following pictogram:
6 The manufacturer of a medical device must demonstrate compliance with the general safety and performance requirements, taking into account the intended purpose (Art. 6 para. 2 MedDO in conjunction with Annex I EU MDR). In the case of a single-use device, the general safety and performance requirements are checked with regard to the single application or use, based on the intended purpose. This means that the single-use device meets the prescribed safety and performance requirements in accordance with Annex I EU MDR for a single application or use – further uses were not tested as part of the conformity assessment procedure (see Art. 21 MedDO) and the manufacturer therefore makes no statement regarding safety and performance in the event of multiple uses.
7 When a used medical device is reprocessed, it is exposed to very high chemical, mechanical and thermal stresses. Since a single-use device is not designed for such treatment, there is a risk that it will be damaged or weakened, so that its proper functioning after reprocessing is no longer guaranteed. In addition to this problem, however, there is also the risk that a single-use product cannot be completely cleaned and sterilized, which can lead to biological residues with a corresponding risk of infection when it is reused. This risk exists in particular with threads or other areas of a single-use product that are difficult to access. If the manufacturer has placed its product on the market as a single-use device, reprocessing and reuse of such a device is not indicated for reasons of health protection and was therefore explicitly prohibited in the revision of the medical device legislation with the introduction of Art. 73 MedDO.
III. Para. 2
8 Art. 17 para. 1 EU MDR allows the (re)processing of single-use devices provided that (a) the national law of a Member State permits this and (b) the conditions of Art. 17 EU MDR are met. Unlike current Swiss law, EU law therefore explicitly allows the (re)processing and reuse of single-use devices. However, to prevent the prohibition in Switzerland from being circumvented by importing such single-use devices that have been reprocessed in a permissible manner under EU law, Art. 73 para. 2 MedDO explicitly prohibits the making available and use of devices that have been reprocessed in accordance with Art. 17 EU MDR.
9 However, it is unclear why Art. 73 para. 2 MedDO, according to the wording, only covers devices that are reprocessed in accordance with Art. 17 para. 3 EU MDR. Para. 3 of Art. 17 EU MDR only deals with a special situation regarding the reprocessing of single-use devices: the reprocessing and use of single-use devices within a (European) healthcare facility. However, Art. 17 para. 3 EU MDR does not cover the general reprocessing of single-use devices – this is regulated by paras. 1 and 2 of Art. 17 EU MDR. The FOPH's statements in the Explanatory Report on the Total Revision of the MedDO and ClinO-MedDO do not address this at all, but generally state that single-use devices that are reprocessed in accordance with the provisions of Art. 17 EU MDR are not marketable in Switzerland. The following is stated verbatim: “Thus, no single-use devices reprocessed under the provisions of the EU MDR are tolerated on the market (import) or for use in Switzerland.” However, these statements by the FOPH do not correspond to the text of the ordinance, which explicitly refers to Art. 17 para. 3 EU MDR and thus basically only covers single-use devices that are reprocessed within a healthcare facility. In view of the objective of Art. 73 para. 2 MedDO – namely to prevent the circumvention of Art. 73 para. 1 MedDO by importing single-use devices that have been reprocessed abroad – a reference should have been made to Art. 17 EU MDR as a whole, without further reference to a specific paragraph of Art. 17 EU MDR. A purely literal interpretation of Art. 73 para. 2 MedDO would therefore lead to the conclusion that single-use devices that have been reprocessed in the European Union in accordance with Art. 17 para. 1 or 2 EU MDR may in principle be used in Switzerland. However, it is to be assumed that the supervisory authorities will not regard this interpretation as decisive, with reference to the spirit and purpose of this provision.
IV. Consequences of non-compliance
10 Non-compliance with Art. 73 MedDO is punishable. Pursuant to Art. 86 para. 1 let. d TPA, anyone who intentionally places on the market, exports or uses medical devices that do not meet the requirements of the TPA shall be punished with a custodial sentence of up to three years or a monetary penalty. In the event of a negligent offense, a monetary penalty is imposed and in minor cases a fine may be imposed (Art. 86 para. 4 TPA). In this context, however, the question arises as to whether the unclear provision in Art. 73 para. 2 MedDO is sufficient as a basis for punishment if single-use devices that have been reprocessed in accordance with Art. 17 para. 1 or 2 EU MDR are used in Switzerland.
11 The legal requirements for medical devices are set out, among other places, in Art. 45 TPA, para. 7 of which is specified in Art. 73 MedDO, which is why the implementing law must also be observed when assessing a violation of the legal requirements.
Bibliography
Suter Benedikt A./Pieles Yvonne, Kommentierung zu Art. 86, in: Eichenberger Thomas/Jaisli Urs/Richli Paul (Hrsg.), Basler Kommentar, Heilmittelgesetz, 2. Aufl., Basel 2022.
Zobrist Markus, Invasive Medizinprodukte für den Einmalgebrauch sind nicht wiederverwendbar, SGSV Forum 2 (2004), S. 31 ff.
Meier Andreas L./Cortizo Juan, Kommentierung zu Art. 45, in: Eichenberger Thomas/Jaisli Urs/Richli Paul (Hrsg.), Basler Kommentar, Heilmittelgesetz, 2. Aufl., Basel 2022.
Materials
Bundesamt für Gesundheit, Erläuternder Bericht zur Totalrevision der Medizinprodukteverordnung und Verordnung über klinische Versuche mit Medizinprodukten, Juli 2020 («BAG, Erläuternder Bericht»).